How Many Atoms Are in 4.5 Moles of Al
3 moles of oxygen needs 4 moles of Al to react. Calculate how many grams of AlOH3 are in 450 moles of AlOH3.
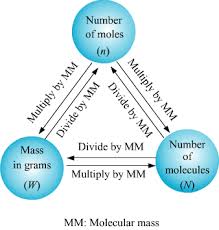
The Mole Concept Chemistry Quiz Quizizz
4 3 6 moles of Al.

. In this case Al2 means 2 aluminums SO43 means 3 SO4s. Use at least three significant figures. Atomic mass of Al 26982 gmol Atomic mass of O 15999 gmol Atomic mass of H 100794 gmol.
How many moles are in 575x10 24 atoms of Al. How many atoms in 45 moles of carbon C. 10 moles to atom 60221415E24 atom.
So 3 moles of oxide were produced by 3. 2 moles of oxide were produced by 4 moles of Al. The parentheses mean that the 3 has to be multiplied by whatever number of atoms you have of each element inside.
Convert grams CaCO3 to moles or moles CaCO3 to grams Molecular weight calculation. 5 moles to atoms 301107075E24 atoms. 1 mole 6022 1023 atomsmolecules.
View the full answer. Therefore the number of moles of Al2O3 is 29125710196 2857 moles. Moles of Cu in 78 x 1023 atoms Cu.
How many moles of H 2 are made from 30 moles of HCl. X 45 moles 6022 10²³ Atoms 1 mole X 270 10²⁴ Atoms of S Result. Find the molar mass of AlOH 3.
This means there is 1 atom of Al. 40078 120107 1599943 Percent composition by element Element Symbol Atomic Mass of Atoms Mass Percent Calcium Ca. 45 Moles of Na₂SO₄ contains 270 10²⁴ Atoms of Sulfur.
Complete Mole Unit Conversions. 1 mole correspond to 60231023 atoms 1 mole of Mg3 PO42 contains 8moles of oxygen atoms 448 moles contains 448835. Calculate the mass in g of 45 1023 CO molecules.
Molatoms 1 mol 602E23 atoms. 9 moles to atom 541992735E24 atom. 40 g 1 mole 602 x 1023 atoms 62 x 1022 atoms.
108 x 1022 molecules. How many aluminum atoms are in 291257 grams of Al2O3. So 4 x 602 x 10 23 power of atoms.
Show your work for partial credit. 8 moles to atom 48177132E24 atom. Calculate the percent composition by mass of each element in aloh3.
How many moles are in 250x10. Number of Li atoms in 45 mol Li. 7 moles to atom 421549905E24 atom.
I get enough amount of oxgen so the limiting reactant is the Al. 10 moles to atoms 60221415E24 atoms. The gram molecular mass of Al2O3 is 226982 3 15999 10196.
Solution for How many moles of aluminum atoms Al are needed to combine with 0332 mol of O atoms to make aluminum oxide Al203. 6 moles to atom 36132849E24 atom. If 45 moles of AlCl 3 are produced what mass of H 2 is also produced.
Atomsumol 1 umol 602E17 atoms. Dividing 269860210 23 yields a mass of 44810 23 g for one aluminum atom. Then 45 moles of oxygen must need 45.
8 moles to atoms 48177132E24 atoms. There is no subscript given to Al. Number of CO2 molecules in 00180 mol CO2.
How many atoms are in 40g of potassium. Show your work for partial credit. Convert 235 moles of carbon to atoms.
3x1 sulfurs 3x4 oxygens. How many moles are. Atom Mole 60221415E23.
Atomsmmol 1 mmol 602E20 atoms. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. How many atoms are in al2 so43.
How many atoms in 45 moles of carbon C. Up to 24 cash back How many atoms are in caco3 Molar mass of CaCO3 1000869 gmol This compound is also known as Calcium Carbonate. 4 2 6 moles of Al.
235 moles 602 x 1023 atoms 141 x 1024 atoms 1 mole 4. 35 Related Question Answers Found. 4 moles to atoms 24088566E24 atoms.
7 moles to atoms 421549905E24 atoms. Each mole has avogadros number of atoms in it which is 602 x10 to the 23rd power. If I use the reactants I get the same value.
A subscript 3 is written outside the parenthesis So multiply the atoms inside the parenthesis by 3. 1 mole of aluminium has 60221023 Al atoms Hence 42 moles has 42 60221023 25291023 or 2531024 Al atoms 1 mole of aluminium has 60221023 Al atoms Hence 42 moles has 42 60221023 25291023 or 2531024 Al atoms. Atomsmol 1 mol 602E23 atoms.
Do you know the correct answer. Chemistry 22012021 1940 httpcindy14. 425 x 1026 atoms of barium 1 mol 706 mol 602 x 1023 atoms 3.
Atomsnmol 1 nmol 602E14 atoms. Experts are tested by Chegg as specialists in their subject area. Atomspmol 1 pmol 602000000000 atoms.
Moles of C2H6 in 3754 x 1023 molecules C2H6. Al 1 O 3 and H 3. Where Atom Number of atoms.
6 moles to atoms 36132849E24 atoms. 5 moles to atom 301107075E24 atom. 27 x 1024 atoms.
We review their content and use your feedback to keep the quality high. What mass of HCl is required to react with 175 mole of Al. Answer 1 of 4.
One mole of aluminum atoms has a mass of 2698 grams and contains 6021023 atoms. Atomsfmol 1 fmol 602000000 atoms. 4 moles to atom 24088566E24 atom.
9 moles to atoms 541992735E24 atoms.
How Many Atoms Are Present In 4 2 Moles Of Aluminum Quora

Embroidery Machine Bilby Mole Pattern Machine Embroidery Patterns Machine Embroidery Projects Machine Embroidery

0 Response to "How Many Atoms Are in 4.5 Moles of Al"
Post a Comment